Phenotypic heterogeneity and the evolution of virulence
Evolution provides the framework for all biological processes, including Salmonella pathogenesis. Building on our initial work on the bistable-gene expression of TTSS-1, we have teamed up with the research groups of Roland Regoes and Martin Ackermann to develop experimental approaches and mathematical models to study single cell virulence gene expression and its consequences for the evolution of Salmonella virulence. This has enabled paradigmatic new insights:
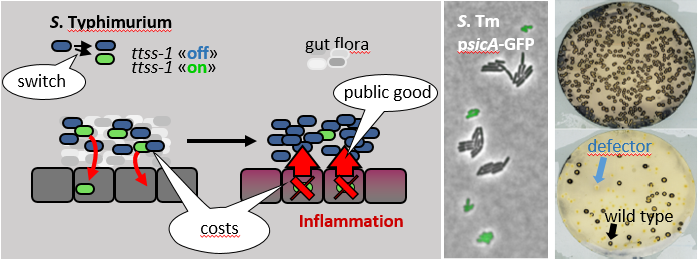
a) S. Typhimurium forms subpopulations of cells which express a virulent or an avirulent phenotype, within the host. These subpopulations cooperate and are necessary for the pathogen's survival and ability to sustain colonization (Ackermann et al. 2008; Diard et al. 2013).
b) The "costs" of virulence factor expression can have profound consequences on the evolution of the pathogen. In fact, the costs of TTSS-1 expression are quite high: TTSS-1 expression reduces the pathogen's growth rate by as much as 2-fold (Sturm et al., 2011) and the bacterial cells that invade into the gut tissue via TTSS-1 are in most cases killed off by the innate immune response within the mucosa (Ackermann et al., 2008). These "costs" are so high that (at least in extreme cases) defector mutants which lost the ability to express TTSS-1 can arise within a few days and replace the wild-type S. Typhimurium in the gut lumen (Diard et al., 2013). Now, we are studying how S. Typhimurium minimizes the costs of virulence factor expression and thus stabilizes its virulent genotype.
We aim to develop experimental approaches to decipher the role of phenotypic heterogeneity of the pathogen and of the host (Kreibich and Hardt, 2015). This will be essential for explaining not only pathogen evolution, but also how an infection proceeds, which cells are fostering disease progression and why some hosts escape an infection (Diard et Hardt, 2017).
Literature
Ackermann, M.*, B. Stecher, N. E. Freed, P. Songhet, W. D. Hardt* and M. Doebeli* (2008) Self-destructive cooperation mediated by phenotypic noise. Nature, 454(7207):987-90. external page[Abstract]call_made
Sturm, A., Heinemann, M., Arnoldini, M., Benecke, A., Ackermann, M., Benz, M., Dormann, J. and W.D. Hardt* (2011) The Cost of Virulence: Retarded Growth of Salmonella Typhimurium Cells Expressing Type III Secretion System 1. PLoS Pathog. 7(7):e1002143. external page[Abstract]call_made
Diard, M., Garcia, V., Maier, L., Remus-Emsermann, M.N.P., Regoes, R.R.*, Ackermann, M.* and W.D. Hardt* (2013) Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature, 494(7437):353-6. external page[Abstract]call_made
Diard, M., Sellin, M.A., Dolowschiak, T., Arnoldini, M., Ackermann, M. and W.D. Hardt* (2014) Antibiotic treatment selects for cooperative virulence of Salmonella Typhimurium. Current Biology, 24(17):2000-5. external page[Abstract]call_made
Kreibich S. and W.D. Hardt* (2015) Experimental approaches to phenotypic diversity in infection. Curr Opin Microbiol. 27:25-36. external page[Article]call_made
Diard, M., Bakkeren, E., Cornuault, J. K., Moor, K., Hausmann, A., Sellin, M. E., Loverdo, C., Aertsen, A., Ackermann, M., De Paepe, M., Slack, E. and Hardt, W.-D. (2017) Inflammation boosts bacteriophage transfer between Salmonella spp. Science, 17 Mar 2017, Vol. 355, Issue 6330, pp. 1211-1215; DOI: 10.1126/science.aaf8451
[external pageAbstractcall_made] [external pageFREE FULL TEXTcall_made] [external pageFREE PDF-REPRINTcall_made]
Diard M., Hardt W. D. (2017) The evolution of bacterial virulence. FEMS Microbiol Rev. 2017 Sep 1;41(5):679-697. external page[Abstract]call_made