Group Wetter Slack
In healthy individuals, the mucosae of the intestine, lungs and genital tract operate robustly to permit the presence of a "commensal" bacterial consortium, while maintaining vigilance to invading pathogens. Mucosae are also common sites for pathogen evolution and horizontal gene transfer. Our group aims to understand exactly how (and to what extent) the host can influence the composition, behaviour and evolution of mucosal bacterial consortia.
Mucosal systems are highly dynamic, typically involving fluid flow over the epithelium, continuous bacterial growth and adaptation, and homeostatic activation of immune mechanisms. Small changes, for example in how quickly bacteria are killed in the intestinal tissues, can therefore translate into very large shifts in immune system activation, and subsequently on selective pressures acting on the microbiota [1].
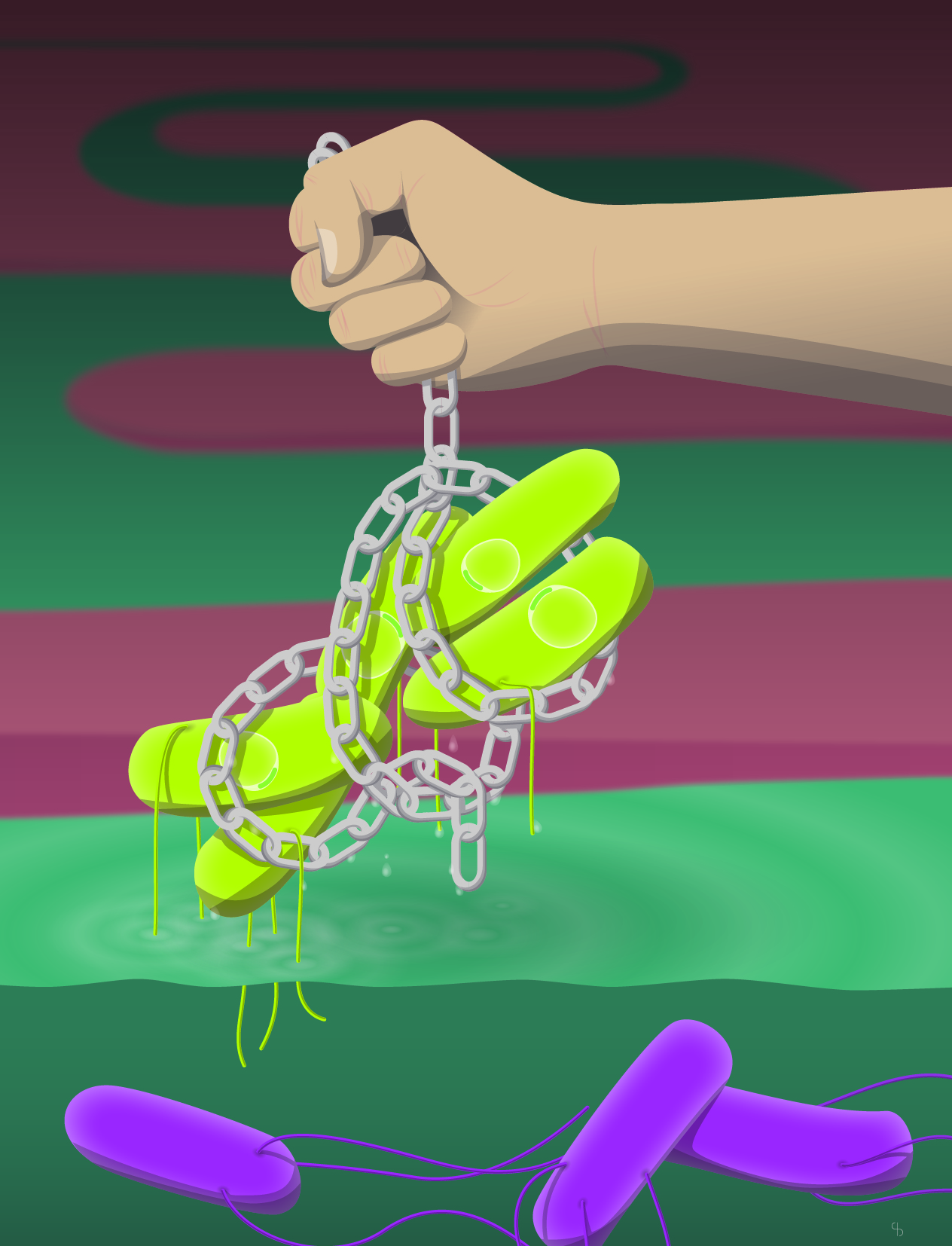
Through a successful collaboration with Dr. D. R. Brumley at the University of Melbourne, as well as biological mathematicians at ETH Zürich and CNRS, Paris, we could study intestinal antibodies as part of such a dynamic system. High-avidity, protective IgA can be induced against most cultivable bacterial species using high-dose peracetic acid-inactivated oral vaccines and can provide protection [2-3]. In non-Typhoidal Salmonellosis, IgA protects by clumping and preventing Salmonella from interacting with the epithelium ("immune exclusion"). However, considering fluid dynamics and typical densities of individual bacterial species in the gut lumen, it became clear that classical agglutination (i.e. collision-dependent bacterial clumping) was not driving this protection. At realistic pathogen densities, the frequency of bacterial collision is simply low. Instead, vaccine-induced IgA cross-links bacteria during cell division, driving the formation on clumps based on bacterial growth [4]. We named this process "enchained growth". Enchained clumps cannot approach the epithelium, preventing disease, and are more rapidly cleared in the fecal stream. As these clumps are formed from the progeny of a single bacterial cell, enchained growth also affects bacterial evolution. Clonal extinction rates increase and horizontal gene transfer via plasmids [4] or phage [5] is blocked.
References
1. Slack, E. et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 325, 617-620, doi:10.1126/science.1172747 (2009).
2. Moor, K. et al. Peracetic acid treatment generates potent inactivated oral vaccines from a broad range of culturable bacterial species. Frontiers in Immunology 7, doi:10.3389/fimmu.2016.00034 (2016).
3. Moor, K. et al. Analysis of bacterial surface-specific antibodies in body fluids using bacterial flow cytometry. Nature Protocols 11, 1531-1553, doi:10.1038/nprot.2016.091 (2016).
4. Moor, K. et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature advance online publication, doi:10.1038/nature22058external pagehttp://www.nature.com/nature/journal/vaop/ncurrent/abs/nature22058.html - supplementary-informationcall_made (2017).
5. Diard, M. et al. Inflammation boosts bacteriophage transfer between Salmonella spp. Science 355, 1211-1215, doi:10.1126/science.aaf8451 (2017).